Medical Monitoring System Tracks Patients’ Health Across In-Hospital Environments
Increasingly, healthcare professionals are enhancing patient care and recovery by monitoring their vital signs with wireless technology. With the Vios Monitoring System (VMS) from Vios Medical, healthcare providers can automate patient oversight and remotely detect problems before they become serious by continuously tracking and analyzing medical-grade vital signs across in-hospital environments.
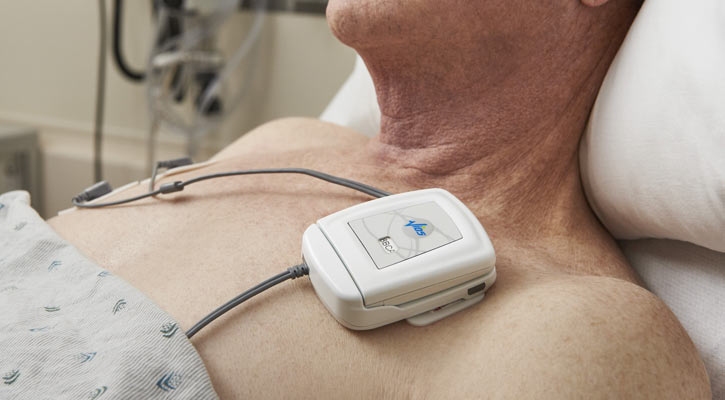
The Vios Monitoring System connects patient-worn sensors, including the Vios Chest Sensor and a commercially available pulse oximeter, with a Bedside Monitor and Central Station Monitor Software to analyze, display and centralize patient data in near real-time. The software can be deployed on off-the-shelf hardware and delivers an engaging user interface that can coordinate care among multidisciplinary teams. The first generation of the Vios Monitoring System has received Food and Drug Administration (FDA) 510(k) clearance but is not currently available in the United States.
Several components on the mobile monitoring device from Vios Medical are injection molded from Bayblend® polycarbonate/acrylonitrile butadiene styrene (PC+ABS) blend and Texin® thermoplastic polyurethane (TPU) supplied by Covestro. The white Bayblend® M301 FR resin is utilized on the mobile monitoring device’s housing and the clear Texin® RxT70A TPU is featured on the hinge, feet, button and seal.
“We needed to utilize materials that would not irritate the patient’s skin, yet were durable enough to protect interior components. The biocompatibility and performance of the selected materials combined with Covestro’s technical support and color matching made them the ideal choice,” said Amit Patel co-founder and CEO of Vios Medical.
Bayblend® M301 FR PC+ABS is ideal for medical devices with housings that require biocompatibility with intact skin. The custom colored polycarbonate has excellent impact resistance and surface appearance to create an aesthetically pleasing device. Bayblend® resin also meets UL requirements. Texin® RxT70A TPU offers a water-tight seal and flexible properties advantageous to the hinge, button and feet of the Vios Medical wearable monitoring device.
“As the demand for wearable medical devices and mobile patient monitoring systems continues to grow, materials must keep up to satisfy the unique needs of these devices. Covestro is dedicated to providing materials that are of the highest quality to be used in life-saving products like the Vios Monitoring System,” Brian Faul, business development manager, Central Midwest region, Polycarbonates, Covestro.
“Our involvement illustrates our commitment to providing innovative solutions to meet important healthcare needs,” said Bruce Fine, market segment leader – medical, North America, Covestro.
Attendees at MD&M West Conference and Exposition, Feb. 9-11 in Anaheim, California, can visit Covestro’s booth (#2021) for more details about Covestro’s material solutions for medical applications.
